The Fascinating Formation of Bismuth Crystals
Share
Bismuth is a metal like no other. It forms stunning, iridescent, geometric crystals that look like something from a sci-fi world. But how does bismuth go from a dull, silvery metal to the mesmerizing rainbow-hued structures that captivate collectors and science enthusiasts alike? Let’s explore the process of how bismuth is formed, both naturally and artificially.
What is Bismuth?
Bismuth (Bi) is a heavy, brittle metal with a pinkish-silver hue in its raw form. It’s one of the few metals that expands when it solidifies, making it unique among its metallic peers. Unlike lead (which it was once mistaken for), bismuth is non-toxic, which has led to its use in cosmetics, pharmaceuticals (like Pepto-Bismol), and even eco-friendly alloys.
How Does Bismuth Form in Nature?
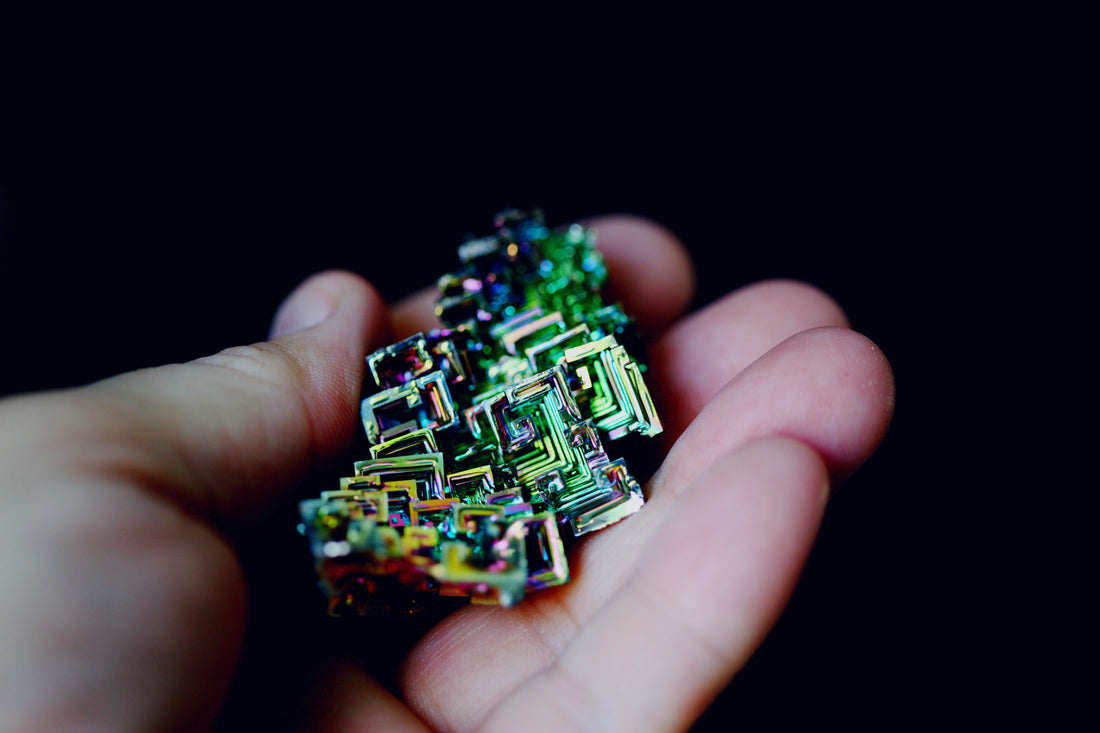
In the Earth’s crust, bismuth is relatively rare and typically found alongside lead, tin, and silver ores. Rather than forming large crystal structures underground, natural bismuth is usually found as small veins or masses in hydrothermal deposits. The naturally occurring form of bismuth doesn’t have the same striking colors as the lab-grown variety—those vibrant hues come from an oxidation process when bismuth is exposed to air.
The Science Behind Artificial Bismuth Crystals
Because natural bismuth is rare and doesn’t easily form large crystals, most of the beautiful specimens you see are created through a controlled melting and cooling process. Here’s how it works:
Step 1: Melting the Metal
Bismuth has a relatively low melting point of about 520°F (271°C), which means it can be melted on a kitchen stove (though handling molten metal requires extreme caution). When heated in a metal pot or crucible, solid bismuth turns into a shimmering, liquid pool.
Step 2: Controlled Cooling
As the molten bismuth cools, the outer edges start to solidify first. If the remaining liquid is carefully poured out, what’s left behind is a hollow, stepped structure. These “staircase” formations occur because of the way bismuth atoms stack as they crystallize.
Step 3: The Magic of Oxidation
Pure bismuth is silvery-pink, but when exposed to air, it forms a thin oxide layer that refracts light, creating a rainbow effect. The colors shift depending on the thickness of the oxide layer, resulting in blues, purples, golds, and greens. This process is similar to how oil on water creates an iridescent sheen.
Why is Bismuth So Popular?
Bismuth’s otherworldly appearance has made it a favorite among collectors, jewelers, and even metaphysical enthusiasts who believe it has grounding and transformation properties. Whether displayed as a raw crystal, made into jewelry, or used in science experiments, bismuth’s unique structure and formation process make it a true wonder of the natural and scientific world.
Would you ever consider melting and growing your own bismuth crystals? It’s a fascinating hands-on experience that blends art and science into one dazzling display!
